A major breakthrough has been announced in the race for a Covid-19 vaccine, with the jab from Pfizer and BioNtech found to be more than 90% effective.
Here is everything you need to know about the coronavirus vaccine race.
– What progress is being made with Covid-19 vaccines?
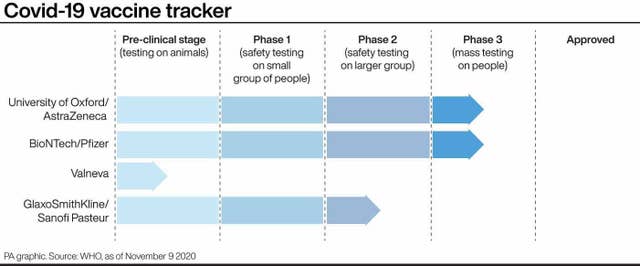
There are currently more than 200 coronavirus vaccine candidates being tested around the world.
About 12 Covid-19 vaccines around the world are currently in the final stages of testing, but the one from German biotech firm BioNtech and US pharmaceutical company Pfizer is the first to report any results.
There are two frontrunners in the Covid-19 vaccine race – the one from Pfizer, called BNT162b2, and another being developed by the University of Oxford and AstraZeneca, which is also in phase three clinical trials.
Other potential vaccines in phase three trials include ones by US drugs firm Moderna and biotech company Novavax.
– How promising are the results from the Pfizer/BioNTech vaccine?
They are interim findings, and studies are set to continue, but analysis shows that the vaccine can prevent more than 90% of people from getting Covid-19.
This is a first but critical step as we continue our work to try to deliver a safe and effective #COVID19 vaccine.
— Pfizer Inc. (@pfizer) November 9, 2020
It has been tested on 43,500 people in six countries and no safety concerns have been raised.
The analysis was carried out after 94 confirmed cases of Covid-19 were found among those taking part in the trial.
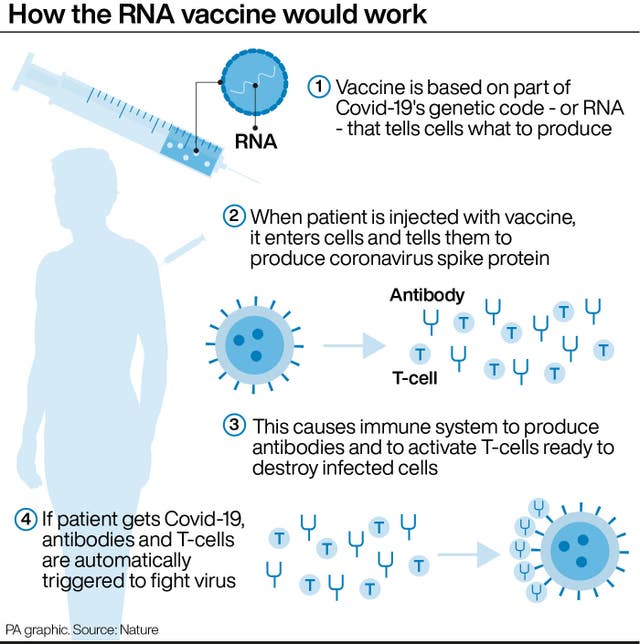
The jab is known as a messenger RNA (mRNA) vaccine, which uses the virus’s genetic code rather than any part of the virus itself, and is injected into the body where it enters cells and tells them to create antigens.
– When can we expect results from the Oxford vaccine?
Over 20,000 volunteers are now participating in trials for the Oxford vaccine, which are taking place in countries including the UK, South Africa, Brazil and Kenya.
Professor Andrew Pollard, head of Oxford’s vaccine trial team, said he is optimistic data on safety and efficacy of their vaccine will be available by the end of the year.
Appearing before the Commons Science and Technology Committee, he said there is a “small chance” of a vaccine being made available by Christmas.
The Oxford vaccine, called ChAdOx1 nCoV-19, uses a weakened version of a common cold virus (adenovirus) which causes infections in chimpanzees.
– What other trials are ongoing in the UK?
Aside from the Oxford vaccine, a coronavirus jab is being developed by Imperial College London.
The Imperial vaccine is in phase one of clinical testing, where doses are being given to a small group of people to determine whether it is safe and to learn more about the immune response it provokes.
Professor Robin Shattock, who is leading Imperial College London’s Covid-19 vaccine effort, said data on its efficacy will be available in the middle of next year.
Pharmaceutical companies Sanofi and GlaxoSmithKline have also teamed up with the hope of making a Covid-19 vaccine available by the middle of next year.
We welcome the encouraging vaccine news from @pfizer & @BioNTech_Group & salute all scientists & partners around the 🌍 who are developing new safe, efficacious tools to beat #COVID19. The 🌍 is experiencing unprecedented scientific innovation & collaboration to end the pandemic!
— Tedros Adhanom Ghebreyesus (@DrTedros) November 9, 2020
The Sanofi/GSK candidate is in the phase two stage, where the vaccine is being given to hundreds of people so scientists can learn more about its safety and correct dosage, and plan to begin phase three by the end of the year.
– Does the UK have access to any of these potential vaccines?
The UK has secured 40 million doses of the Pfizer/BioNTech vaccine, the first agreement the firms signed with any government.
In August, the Government announced the UK has secured access to six Covid-19 vaccine candidates in development, representing 340 million doses.
The deals cover four different types of vaccines – adenoviral vaccines, mRNA vaccines, inactivated whole virus vaccines and protein adjuvant vaccines.
Adenoviral vaccines are weakened versions of adenoviruses, while mRNA candidates use the virus’s genetic code, as with the Pfizer/BioNTech jab.
Inactivated whole virus vaccines, on the other hand, contain whole bacteria or viruses which have been killed, while protein adjuvant jabs are those where an adjuvant is added to enhance the immune response.
– When could a vaccine become available in the UK?
A vaccine usually takes years, often decades, to develop, but scientists working on potential coronavirus jabs are hoping to achieve the same amount of work in a few months.
Pfizer and BioNTech plan to apply to the US Food and Drug Administration – the US medicines regulator – by the end of this month for emergency approval to use the vaccine.
Downing Street welcomed the results as “promising” and said the UK will have procured 10 million doses by the end of the year to be given out if it is approved.
NHS England chief executive Sir Simon Stevens said the “expectation” is that any vaccination programme would begin in the new year – pending positive results from clinical trials.
Kate Bingham, chairwoman of the UK vaccine taskforce, said she has 50% confidence that by Easter or early summer next year, all vulnerable people in the country will have a vaccine.




Comments: Our rules
We want our comments to be a lively and valuable part of our community - a place where readers can debate and engage with the most important local issues. The ability to comment on our stories is a privilege, not a right, however, and that privilege may be withdrawn if it is abused or misused.
Please report any comments that break our rules.
Read the rules hereLast Updated:
Report this comment Cancel